Second Law of Thermodynamics
Second Law of Thermodynamics: Overview
This topic covers concepts such as second law of thermodynamics and forms of second law of thermodynamics.
Important Questions on Second Law of Thermodynamics
Given the following entropy values at 298 K and 1 atm : The entropy change for the reaction
A flask containing at . and was connected to a flask containing gas at and . The gases were allowed to mix isothermally, determine the entropy change for the system:
In an experiment, of water, initially at is heated to via two processes. In process I, water is brought into contact with a heat reservoir at . In process II, water is first brought into contact with a heat reservoir at and then in contact with another reservoir at . Assume that the specific heat capacity of water, is independent of temperature. Choose the correct options from the following. (Note )
An ice cube of mass at is left sitting on the kitchen table where it gradually melts and reaches the kitchen temperature of . The latent heat of fusion for ice is and the specific heat of water is . Select the correct statement(s) about this process. (All numerical values in the answers are rounded to the closest integer.)
Two solid blocks at temperatures and are put in thermal contact for a long time inside an insulated container. Choose the correct statement(s).
Heat cannot by itself flow from a body at lower temperature to a body at higher temperature” is a statement or consequence of
Write the statement of second law of thermodynamics
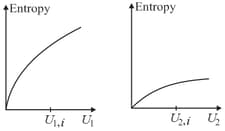
Graphs below show the entropy versus energy of two systems and at constant volume. The initial energies of the systems are indicated by and respectively. Graphs are drawn to the same scale. The systems are then brought into thermal contact with each other. Assume that, at all times the combined energy of the two systems remains constant. Choose the most appropriate option indicating the energies of the two systems and the total entropy after they achieve the equilibrium.
When of ice at melts to water at , the resulting change in its entropy, taking latent heat of ice to be , is,
The change in entropy when a ice cube at is transformed into water at in is
Take ,
Heat engine is a device in which a system undergoes a _____ process resulting in conversion of heat into work. (cyclic/non-cyclic)
_____ in thermodynamics refers to the situation when macroscopic variables describing the thermodynamic state of a system do not depend on time.
One mole of an ideal monatomic gas undergoes the following four reversible processes:
Step It is first compressed adiabatically from volume to
Step then expanded isothermally to volume
Step then expanded adiabatically to volume
Step then compressed isothermally to volume If the efficiency of the above cycle is then is
What is the entropy change in during the melting of 27.3 g of ice of ? (Latent heat of fusion of ice = 330 )
Choose the incorrect relation among these
According to the third law of thermodynamics which one of the following quantities for a perfectly crystalline solid is zero at absolute zero?
In the following a statement of Assertion is followed by a statement of Reason.
Assertion: During free expansion of an ideal gas, its entropy increases but work done by the gas is zero.
Reason: During free expansion, volume increases but temperature remains constant.
Which of the following statements is true? The entropy of the universe
Molar heat of vaporisation of a liquid is . If the entropy change is , the boiling point of the liquid is
One mole of a perfect gas expands isothermally to ten time of its original volume. The change in entropy is
